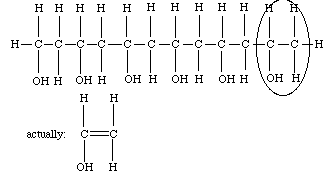Experiment 2
Slime Away
Cross-Linking Poly (vinyl alcohol) with Sodium Borate
Objective: The objective of this experiment is to explore the change in physical properties of a polymer as a result of cross-linking. The result of adding more cross-linking agents to a polymer is considered and another model of cross-linking is viewed.
Applications:
There are a number of uses of the PVA polymer we are studying:
- They may be used in sheets to make bags to act as containers for pre-measured soap you simply throw into a washing machine.
- The PVA sheets may be made into larger bags to be used by hospitals as containers for the cotton cloth used in the operating rooms or to hold the bed linen or clothing of infected patients.
Time: This experiment will require approximately 15-20 minutes to run and clean up.
Materials and Supplies:
- 100 ml/group of poly (vinyl alcohol) 4%
- 10 ml of sodium borate 4%
- Styrofoam cups and wooden stir sticks (tongue depressors)
- Zip lock bags or latex gloves (surgical)
General Safety Guidelines:
- Laboratory aprons and goggles should be worn in this experiment as in all procedures.
- Both the borax and the PVA will burn the eyes. Hands should be washed at the end of the experiment.
Procedure:
The polyvinyl alcohol and sodium borate are mixed together in approximately a 10 to 1 ratio.
- 100 ml of the 4% poly (vinyl alcohol) is added to a Styrofoam cup .
- Food coloring can be added to the PVA in the cups to make different colors. Simple food coloring is recommended. This coloring should be added before any of the borax solution has been added, or it can be added directly to the borax solution.
- Add 10 ml of the 4% cross-linker (sodium borate) to each cup. Begin stirring the mixture immediately with your wooden tongue depressor.
- Make observations as to what is occurring as the reaction proceeds.
- Within a couple of minutes the slime will be formed. Lift some of it out with the tongue depressor and make your observations. Record your observations on your data sheet.
- Take some in your hand and stretch the slime slowly. Record your observations on your data sheet.
- Repeat the stretching exercise only this time do it rapidly. Record your observations on your data sheet. Compare the results of the two tests. The slime is non toxic and is safe to handle, so you can put it in a Zip-lock bag (or latex glove) and seal it to take home.
- Follow good laboratory procedure and wash your hands with soap and water. It is recommended that this procedure be followed whenever handling this material. Keep it in the glove or bag until it is discarded. The sodium borate or PVA could burn your eyes.
- Place a small amount of the PVA on a paper towel and set it off to the side to dry until tomorrow. Upon returning to class the next day, record in the data section your observation of the slime.
Data and Analysis:
Observation of the PVA before the sodium borate is added:
Observation of the PVA after the sodium borate is added:
Observation of stretching the cross-linked PVA slowly:
Observation of stretching the cross-linked PVA rapidly:
Observation of the cross-linked PVA left out in the air overnight:
Questions:
- What are the physical properties that change as a result of the addition of sodium borate to the poly (vinyl alcohol).
- What would be the effect of adding more sodium borate to your cup (your thoughts only)?
- After making the observations on the dried PVA, how does the water affect the elasticity of the polymer? What is elasticity?
- Find and circle the repeat unit in the polymer molecule below?
- What is the formula of the poly (vinyl alcohol) monomer circled above? (Your teacher may want to show you how to alter this slightly after you have drawn the structure.)
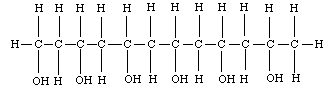
- In the picture below, circle the borax cross-linking agent.
Teacher Notes:
Objective: The objective of this experiment is to explore the change in physical properties as a result of cross-linking polymers. The results of the addition of more cross-linking agents are considered and another model of cross-linking is viewed. Students also have an opportunity for monomer identification.
Experimental:
- The Polyvinyl Alcohol as a solid is mixed in water to make a 4% solution. That is 40.0 grams of PVA per 960 grams (milliliters) of water. The best results are obtained by heating the water to about 80oC on a hot plate with magnetic stirrer. Sprinkle the PVA powder in very gently and slowly on the top of the solution while stirring so as not to cause the mixture to clump together. Temperatures above 90oC may result in decomposition of the PVA and perhaps the creation of an odor to the solution. Continue to sprinkle the PVA into the hot solution while it is stirring. After all of the PVA has been added to the water, place a top on the vessel. If the water evaporates off, a skin of PVA will form. This PVA sheet might also be a nice item to lift off and show the students. Continue stirring until the mixture is uniform (note also that it will be somewhat viscous). Allow the solution to cool, and the resulting solution will be ready for the students to use.
- If students are adding a dye to their PVA, make sure they do this before the addition of borax.
- The borax (sodium borate) can be obtained from your grocery store as "Twenty Mule Team Borax," a laundry bleaching agent. The borax is mixed at a 4% concentration in water. To do this measure out 4 grams of borax and dissolve in 96 grams (milliliters) of water (note: Water has a density of 1 g/mL).
- The material becomes more viscous as we mix the PVA and the borax. It will reach a maximum level of viscosity and will not thicken further without more cross-linking agent. The addition of a higher ratio of Borax will result in a very viscous polymer (like Jell-O).
Theoretical:
The polymer used is "poly (vinyl alcohol)". The monomer has a formula of:
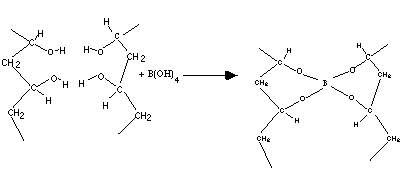
- Borax is sodium borate, Na3BO3. The borax actually dissolves to form boric acid, H3BO3. This boric acid-borate solution is a buffer with a pH of about 9 (basic). Boric acid will accept a hydroxide OH- from water as indicated on the next page.
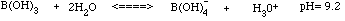
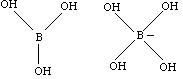
The hydrolyzed molecule will then act in a condensation reaction with PVA as indicated in the last question on the student laboratory.
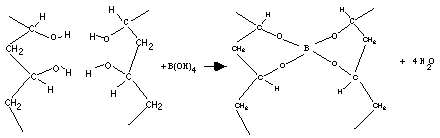
- In the above illustration, two PVA molecules are shown being cross-linked by a hydrated borax molecule. Four molecules of water are also produced.
- The resulting material is about 95% water. It is the water that gives the polymer flexibility. Note that as the polymer dries it returns to its solid phase now as a sheet that is rigid and almost transparent.
- The PVA does not dissolve easily in water. Prepare the PVA solution at least one day in advance.
- Guar Gum dissolves in water much more easily than PVA, but seems to "jell" at a much more unpredictable rate than the PVA mixture does. For this reason, PVA is preferred.
Additional reading for more in depth information can be found in:
Journal of Chemical Education, Jan. 1986, #63, pp. 57-60.
Sample Data and Analysis:
Observation of the PVA before the sodium borate is added:
The solution is fluid.
Observation of the PVA after the sodium borate is added:
The mixture becomes more viscous (thicker).
Observation of stretching the cross-linked PVA slowly:
The slime flows and stretches.
Observation of stretching the cross-linked PVA rapidly:
The slime breaks.
Observation of the cross-linked PVA left out in the air overnight:
It became a dry film.
Answers to Questions:
- The mixture becomes more viscous (thicker).
- The mixture would jell.
- The ability of the cross-linked polymer to stretch decreases. The polymer becomes more brittle and will break.
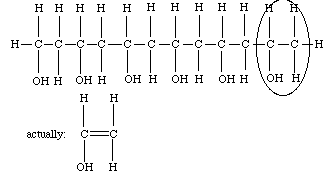
- C2H3OH
- The hydrated borax, minus the four hydrogens are shown on the previous page bonding two chains of the PVA polymer together.