Test Tube Geology
Corrosion of Iron
Objective: The objective of this experiment is to observe over a period of several days the corrosion of iron nails in a test tube.
Time Required: The initial set up will require fifteen to twenty minutes which includes time to mass the copper(II) sulfate. Five to ten minutes each day can then be spent recording observations until no more changes are observed.
Materials and Supplies:
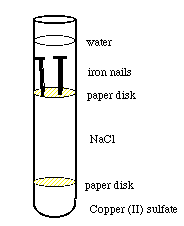
2.5 to 5.0 g CuSO4. 5H2O
NaCl (twice as much volume as that of copper (II) sulfate)
two circles of filter paper or paper toweling
2 to 3 small nails
test tube
test tube rack
long stirring rod
General Safety Guidelines:
Procedure:
Diagram of Set-up:

Questions:
1. Which is the more active metal, sodium or iron?
2. Which is the more active metal, copper or iron?
3. Write the reaction which allows the iron to corrode.
Answers to Questions:
1. Sodium is the more active metal. The iron does not react with the sodium.
2. Iron is more active than copper. The iron is oxidized and the copper is reduced.
3. 2Fe + 3Cu++ --> 2Fe+++ + 3Cu