NOTE: Caution the students against stirring the solution when they put their hand into the water. Mixing will destroy the gradient you have set up and burn them.
Objective: Students will observe the process of forming a solar pond and understand the use of solar heat generated from a solar pond.
Review of Scientific Principles:
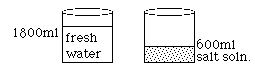
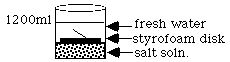
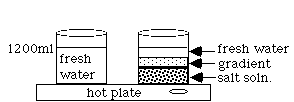
A solar pond uses the principles of energy transfer by convection to heat water. The bottom of the pond is lined with a dark material that absorbs the sun's rays. The water above the liner consists of three layers of varying salt concentrations. The bottom is very salty. The middle layer changes from salty to fresh. The top layer is fresh water. Due to the difference in densities of the three zones, the gradient zone acts as an insulator, so it doesn't allow heat to be passed by convection currents from the saturated middle layer to the fresh water layer. To be more explicit, the gradient zone is a non-convective layer. The bottom layer is a supersaturated salt solution that reaches temperatures of 60-80o C and has convection currents. The top layer of cool fresh water is also convective due to daily heating and cooling from the sun and the wind. The convection currents circulate only above and below the concentration gradient. Cool water is pumped through a pipe placed into the hot supersaturated salt zone. There the water is heated as it passes through the surrounding salt water. From there, the water may be pumped through surrounding homes or barns for heat, or it may be converted to steam for electricity production.
Materials and Supplies:
Procedure:



NOTE: Caution the students against stirring the solution when they put their hand into the water. Mixing will destroy the gradient you have set up and burn them.
Suggestion to teachers: Time permitting, this procedure would make a good laboratory experiment. Or, alternatively, having a few students set this up would be instructive.