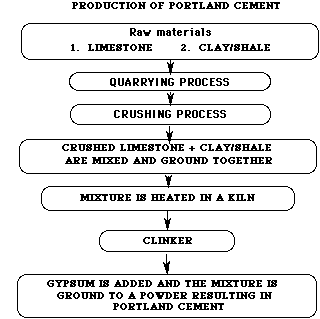
Figure 1: A flow diagram of Portland Cement production.
The importance of concrete in modern society cannot be overestimated. Look around you and you will find concrete structures everywhere such as buildings, roads, bridges, and dams. There is no escaping the impact concrete makes on your everyday life. So what is it?
Concrete is a composite material which is made up of a filler and a binder. The binder (cement paste) "glues" the filler together to form a synthetic conglomerate. The constituents used for the binder are cement and water, while the filler can be fine or coarse aggregate. The role of these constituents will be discussed in this section.
Cement, as it is commonly known, is a mixture of compounds made by burning limestone and clay together at very high temperatures ranging from 1400 to 1600 [[ring]]C.
Although there are other cements for special purposes, this module will focus solely on portland cement and its properties. The production of portland cement begins with the quarrying of limestone, CaCO3. Huge crushers break the blasted limestone into small pieces. The crushed limestone is then mixed with clay (or shale), sand, and iron ore and ground together to form a homogeneous powder. However, this powder is microscopically heterogeneous. (See flowchart.)

The mixture is heated in kilns that are long rotating steel cylinders on an incline. The kilns may be up to 6 meters in diameter and 180 meters in length. The mixture of raw materials enters at the high end of the cylinder and slowly moves along the length of the kiln due to the constant rotation and inclination. At the low end of the kiln, a fuel is injected and burned, thus providing the heat necessary to make the materials react. It can take up to 2 hours for the mixture to pass through the kiln, depending upon the length of the cylinder.
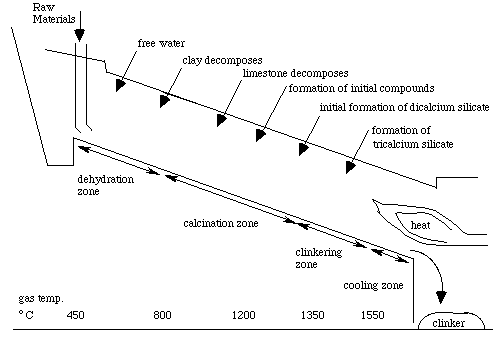
As the mixture moves down the cylinder, it progresses through four stages of transformation. Initially, any free water in the powder is lost by evaporation. Next, decomposition occurs from the loss of bound water and carbon dioxide. This is called calcination. The third stage is called clinkering. During this stage, the calcium silicates are formed. The final stage is the cooling stage.
The marble-sized pieces produced by the kiln are referred to as clinker. Clinker is actually a mixture of four compounds which will be discussed later. The clinker is cooled, ground, and mixed with a small amount of gypsum (which regulates setting) to produce the general-purpose portland cement.
Water is the key ingredient, which when mixed with cement, forms a paste that binds the aggregate together. The water causes the hardening of concrete through a process called hydration. Hydration is a chemical reaction in which the major compounds in cement form chemical bonds with water molecules and become hydrates or hydration products. Details of the hydration process are explored in the next section. The water needs to be pure in order to prevent side reactions from occurring which may weaken the concrete or otherwise interfere with the hydration process. The role of water is important because the water to cement ratio is the most critical factor in the production of "perfect" concrete. Too much water reduces concrete strength, while too little will make the concrete unworkable. Concrete needs to be workable so that it may be consolidated and shaped into different forms (i.e.. walls, domes, etc.). Because concrete must be both strong and workable, a careful balance of the cement to water ratio is required when making concrete.
Aggregates are chemically inert, solid bodies held together by the cement. Aggregates come in various shapes, sizes, and materials ranging from fine particles of sand to large, coarse rocks. Because cement is the most expensive ingredient in making concrete, it is desirable to minimize the amount of cement used. 70 to 80% of the volume of concrete is aggregate keeping the cost of the concrete low. The selection of an aggregate is determined, in part, by the desired characteristics of the concrete. For example, the density of concrete is determined by the density of the aggregate. Soft, porous aggregates can result in weak concrete with low wear resistance, while using hard aggregates can make strong concrete with a high resistance to abrasion.
Aggregates should be clean, hard, and strong. The aggregate is usually washed to remove any dust, silt, clay, organic matter, or other impurities that would interfere with the bonding reaction with the cement paste. It is then separated into various sizes by passing the material through a series of screens with different size openings.
Refer to Demonstration 1
| class | examples of aggregates used | uses |
|---|---|---|
| ultra-lightweight | vermiculite ceramic spheres perlite | lightweight concrete which can be sawed or nailed, also for its insulating properties |
| lightweight | expanded clay shale or slate crushed brick | used primarily for making lightweight concrete for structures, also used for its insulating properties. |
| normal weight | crushed limestone sand river gravel crushed recycled concrete | used for normal concrete projects |
| heavyweight | steel or iron shot steel or iron pellets | used for making high density concrete for shielding against nuclear radiation |
Refer to Demonstration 2
The choice of aggregate is determined by the proposed use of the concrete. Normally sand, gravel, and crushed stone are used as aggregates to make concrete. The aggregate should be well-graded to improve packing efficiency and minimize the amount of cement paste needed. Also, this makes the concrete more workable.
Refer to Demonstration 3
Properties of Concrete
Concrete has many properties that make it a popular construction material. The correct proportion of ingredients, placement, and curing are needed in order for these properties to be optimal.
Good-quality concrete has many advantages that add to its popularity. First, it is economical when ingredients are readily available. Concrete's long life and relatively low maintenance requirements increase its economic benefits. Concrete is not as likely to rot, corrode, or decay as other building materials. Concrete has the ability to be molded or cast into almost any desired shape. Building of the molds and casting can occur on the work-site which reduces costs.
Concrete is a non-combustible material which makes it fire-safe and able withstand high temperatures. It is resistant to wind, water, rodents, and insects. Hence, concrete is often used for storm shelters.
Concrete does have some limitations despite its numerous advantages. Concrete has a relatively low tensile strength (compared to other building materials), low ductility, low strength-to-weight ratio, and is susceptible to cracking. Concrete remains the material of choice for many applications regardless of these limitations.
Hydration of Portland Cement
Concrete is prepared by mixing cement, water, and aggregate together to make a workable paste. It is molded or placed as desired, consolidated, and then left to harden. Concrete does not need to dry out in order to harden as commonly thought.
The concrete (or specifically, the cement in it) needs moisture to hydrate and cure (harden). When concrete dries, it actually stops getting stronger. Concrete with too little water may be dry but is not fully reacted. The properties of such a concrete would be less than that of a wet concrete. The reaction of water with the cement in concrete is extremely important to its properties and reactions may continue for many years. This very important reaction will be discussed in detail in this section.
Portland cement consists of five major compounds and a few minor compounds. The composition of a typical portland cement is listed by weight percentage in Table 2.
| Cement Compound | Weight Percentage | Chemical Formula |
|---|---|---|
| Tricalcium silicate | 50 % | Ca3SiO5 or 3CaO.SiO2 |
| Dicalcium silicate | 25 % | Ca2SiO4 or 2CaO.SiO2 |
| Tricalcium aluminate | 10 % | Ca3Al2O6 or 3CaO .Al2O3 |
| Tetracalcium aluminoferrite | 10 % | Ca4Al2Fe2O10 or 4CaO.Al2O3.Fe2O3 |
| Gypsum | 5 % | CaSO4.2H2O |
When water is added to cement, each of the compounds undergoes hydration and contributes to the final concrete product. Only the calcium silicates contribute to strength. Tricalcium silicate is responsible for most of the early strength (first 7 days). Dicalcium silicate, which reacts more slowly, contributes only to the strength at later times. Tricalcium silicate will be discussed in the greatest detail.
The equation for the hydration of tricalcium silicate is given by:
Tricalcium silicate + Water--->Calcium silicate hydrate+Calcium hydroxide + heat
2 Ca3SiO5 + 7 H2O ---> 3 CaO.2SiO2.4H2O + 3 Ca(OH)2 + 173.6kJ
Upon the addition of water, tricalcium silicate rapidly reacts to release calcium ions, hydroxide ions, and a large amount of heat. The pH quickly rises to over 12 because of the release of alkaline hydroxide (OH-) ions. This initial hydrolysis slows down quickly after it starts resulting in a decrease in heat evolved.
The reaction slowly continues producing calcium and hydroxide ions until the system becomes saturated. Once this occurs, the calcium hydroxide starts to crystallize. Simultaneously, calcium silicate hydrate begins to form. Ions precipitate out of solution accelerating the reaction of tricalcium silicate to calcium and hydroxide ions. (Le Chatlier's principle). The evolution of heat is then dramatically increased.
The formation of the calcium hydroxide and calcium silicate hydrate crystals provide "seeds" upon which more calcium silicate hydrate can form. The calcium silicate hydrate crystals grow thicker making it more difficult for water molecules to reach the unhydrated tricalcium silicate. The speed of the reaction is now controlled by the rate at which water molecules diffuse through the calcium silicate hydrate coating. This coating thickens over time causing the production of calcium silicate hydrate to become slower and slower.
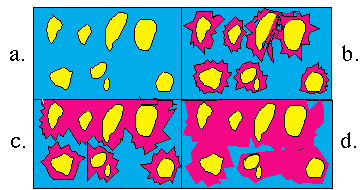
Figure 3: Schematic illustration of the pores in calcium silicate through different stages of hydration.
The above diagrams represent the formation of pores as calcium silicate hydrate is formed. Note in diagram (a) that hydration has not yet occurred and the pores (empty spaces between grains) are filled with water. Diagram (b) represents the beginning of hydration. In diagram (c), the hydration continues. Although empty spaces still exist, they are filled with water and calcium hydroxide. Diagram (d) shows nearly hardened cement paste. Note that the majority of space is filled with calcium silicate hydrate. That which is not filled with the hardened hydrate is primarily calcium hydroxide solution. The hydration will continue as long as water is present and there are still unhydrated compounds in the cement paste.
Dicalcium silicate also affects the strength of concrete through its hydration. Dicalcium silicate reacts with water in a similar manner compared to tricalcium silicate, but much more slowly. The heat released is less than that by the hydration of tricalcium silicate because the dicalcium silicate is much less reactive. The products from the hydration of dicalcium silicate are the same as those for tricalcium silicate:
Dicalcium silicate + Water--->Calcium silicate hydrate + Calcium hydroxide +heat
2 Ca2SiO4 + 5 H2O---> 3 CaO.2SiO2.4H2O + Ca(OH)2 + 58.6 kJ
The other major components of portland cement, tricalcium aluminate and tetracalcium aluminoferrite also react with water. Their hydration chemistry is more complicated as they involve reactions with the gypsum as well. Because these reactions do not contribute significantly to strength, they will be neglected in this discussion. Although we have treated the hydration of each cement compound independently, this is not completely accurate. The rate of hydration of a compound may be affected by varying the concentration of another. In general, the rates of hydration during the first few days ranked from fastest to slowest are:
tricalcium aluminate > tricalcium silicate > tetracalcium aluminoferrite > dicalcium silicate.
Refer to Demonstration 4
Heat is evolved with cement hydration. This is due to the breaking and making of chemical bonds during hydration. The heat generated is shown below as a function of time.
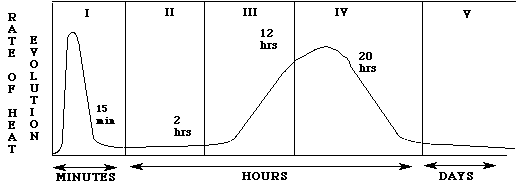
Figure 4: Rate of heat evolution during the hydration of portland cement
The stage I hydrolysis of the cement compounds occurs rapidly with a temperature increase of several degrees. Stage II is known as the dormancy period. The evolution of heat slows dramatically in this stage. The dormancy period can last from one to three hours. During this period, the concrete is in a plastic state which allows the concrete to be transported and placed without any major difficulty. This is particularly important for the construction trade who must transport concrete to the job site. It is at the end of this stage that initial setting begins. In stages III and IV, the concrete starts to harden and the heat evolution increases due primarily to the hydration of tricalcium silicate. Stage V is reached after 36 hours. The slow formation of hydrate products occurs and continues as long as water and unhydrated silicates are present.
Refer to Demonstration 5
Strength of Concrete
The strength of concrete is very much dependent upon the hydration reaction just discussed. Water plays a critical role, particularly the amount used. The strength of concrete increases when less water is used to make concrete. The hydration reaction itself consumes a specific amount of water. Concrete is actually mixed with more water than is needed for the hydration reactions. This extra water is added to give concrete sufficient workability. Flowing concrete is desired to achieve proper filling and composition of the forms. The water not consumed in the hydration reaction will remain in the microstructure pore space. These pores make the concrete weaker due to the lack of strength-forming calcium silicate hydrate bonds. Some pores will remain no matter how well the concrete has been compacted.
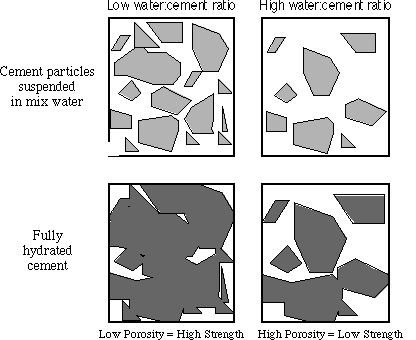
Figure 5: Schematic drawings to demonstrate the relationship between the water/cement ratio and porosity.
The empty space (porosity) is determined by the water to cement ratio. The relationship between the water to cement ratio and strength is shown in the graph that follows.
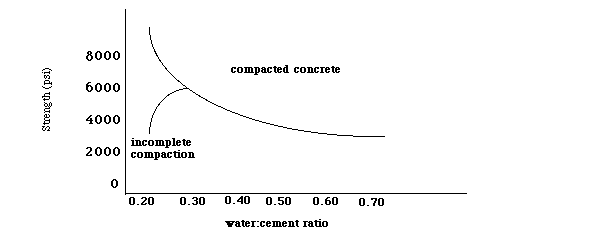
Figure 6: A plot of concrete strength as a function of the water to cement ratio.
Low water to cement ratio leads to high strength but low workability. High water to cement ratio leads to low strength, but good workability.
The physical characteristics of aggregates are shape, texture, and size. These can indirectly affect strength because they affect the workability of the concrete. If the aggregate makes the concrete unworkable, the contractor is likely to add more water which will weaken the concrete by increasing the water to cement mass ratio.
Time is also an important factor in determining concrete strength. Concrete hardens as time passes. Why? Remember the hydration reactions get slower and slower as the tricalcium silicate hydrate forms. It takes a great deal of time (even years!) for all of the bonds to form which determine concrete's strength. It is common to use a 28-day test to determine the relative strength of concrete.
Concrete's strength may also be affected by the addition of admixtures. Admixtures are substances other than the key ingredients or reinforcements which are added during the mixing process. Some admixtures add fluidity to concrete while requiring less water to be used. An example of an admixture which affects strength is superplasticizer. This makes concrete more workable or fluid without adding excess water. A list of some other admixtures and their functions is given below. Note that not all admixtures increase concrete strength. The selection and use of an admixture are based on the need of the concrete user.
SOME ADMIXTURES AND FUNCTIONS
| TYPE | FUNCTION |
|---|---|
| AIR ENTRAINING | improves durability, workability, reduces bleeding, reduces freezing/thawing problems (e.g. special detergents) |
| SUPERPLASTICIZERS | increase strength by decreasing water needed for workable concrete (e.g. special polymers) |
| RETARDING | delays setting time, more long term strength, offsets adverse high temp. weather (e.g. sugar ) |
| ACCELERATING | speeds setting time, more early strength, offsets adverse low temp. weather (e.g. calcium chloride) |
| MINERAL ADMIXTURES | improves workability, plasticity, strength (e.g. fly ash) |
| PIGMENT | adds color (e.g. metal oxides) |
Table 3: A table of admixtures and their functions.
Durability is a very important concern in using concrete for a given application. Concrete provides good performance through the service life of the structure when concrete is mixed properly and care is taken in curing it. Good concrete can have an infinite life span under the right conditions. Water, although important for concrete hydration and hardening, can also play a role in decreased durability once the structure is built. This is because water can transport harmful chemicals to the interior of the concrete leading to various forms of deterioration. Such deterioration ultimately adds costs due to maintenance and repair of the concrete structure. The contractor should be able to account for environmental factors and produce a durable concrete structure if these factors are considered when building concrete structures.
Concrete Summary
Concrete is everywhere. Take a moment and think about all the concrete encounters you have had in the last 24 hours. All of these concrete structures are created from a mixture of cement and water with added aggregate. It is important to distinguish between cement and concrete as they are not the same. Cement is used to make concrete!
(cement + water) + aggregate = concrete
Cement is made by combining a mixture of limestone and clay in a kiln at 1450[[ring]] C. The product is an intimate mixture of compounds collectively called clinker. This clinker is finely ground into the powder form. The raw materials used to make cement are compounds containing some of the earth's most abundant elements, such as calcium, silicon, aluminum, oxygen, and iron.
Water is a key reactant in cement hydration. The incorporation of water into a substance is known as hydration. Water and cement initially form a cement paste that begins to react and harden (set). This paste binds the aggregate particles through the chemical process of hydration. In the hydration of cement, chemical changes occur slowly, eventually creating new crystalline products, heat evolution, and other measurable signs.
cement + water = hardened cement paste
The properties of this hardened cement paste, called binder, control the properties of the concrete. It is the inclusion of water (hydration) into the product that causes concrete to set, stiffen, and become hard. Once set, concrete continues to harden (cure) and become stronger for a long period of time, often up to several years.
The strength of the concrete is related to the water to cement mass ratio and the curing conditions. A high water to cement mass ratio yields a low strength concrete. This is due to the increase in porosity (space between particles) that is created with the hydration process. Most concrete is made with a water to cement mass ratio ranging from 0.35 to 0.6.
Aggregate is the solid particles that are bound together by the cement paste to create the synthetic rock known as concrete. Aggregates can be fine, such as sand, or coarse, such as gravel. The relative amounts of each type and the sizes of each type of aggregate determines the physical properties of the concrete.
sand + cement paste = mortar
mortar + gravel = concrete
Sometimes other materials are incorporated into the batch of concrete to create specific characteristics. These additives are called admixtures. Admixtures are used to: alter the fluidity (plasticity) of the cement paste; increase (accelerate) or decrease (retard) the setting time; increase strength (both bending and compression); or to extend the life of a structure. The making of concrete is a very complex process involving both chemical and physical changes. It is a material of great importance in our lives.